PolyPeptide provides its offering through its manufacturing sites and with a “start here – stay here” philosophy, covering the entire life cycle of a drug, starting with the customer’s pre-clinical drug development projects, followed by clinical phases through to commercialization. As a result, its customer relationships are often strategic and long-term by nature.
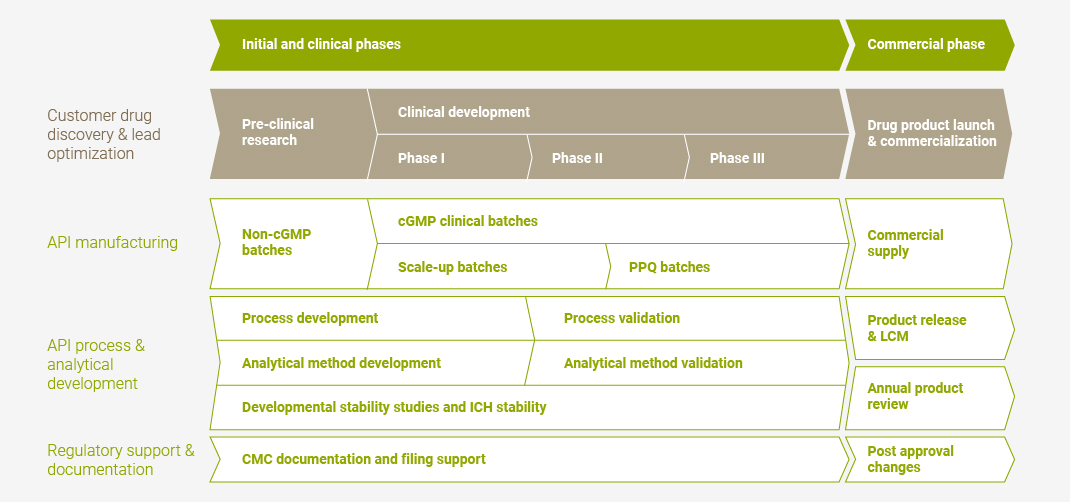
API – Active Pharmaceutical Ingredient; CMC – Chemistry, Manufacturing & Controls; cGMP – current Good Manufacturing Practice; ICH – International Council for Harmonization; LCM – Life Cycle Management; NDA – New Drug Application; PPQ – Process Performance Qualification.

